PhD student discovers that a cell type previously thought to be a source of nutrients can make an embryo on its own
Madeleine Linneberg-Agerholm, and colleagues in the Brickman group at the Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW, University of Copenhagen, have had a paper published in Cell following the discovery of the remarkable memory contained within a cell type previously thought to be a source of nutrients for the early embryo.
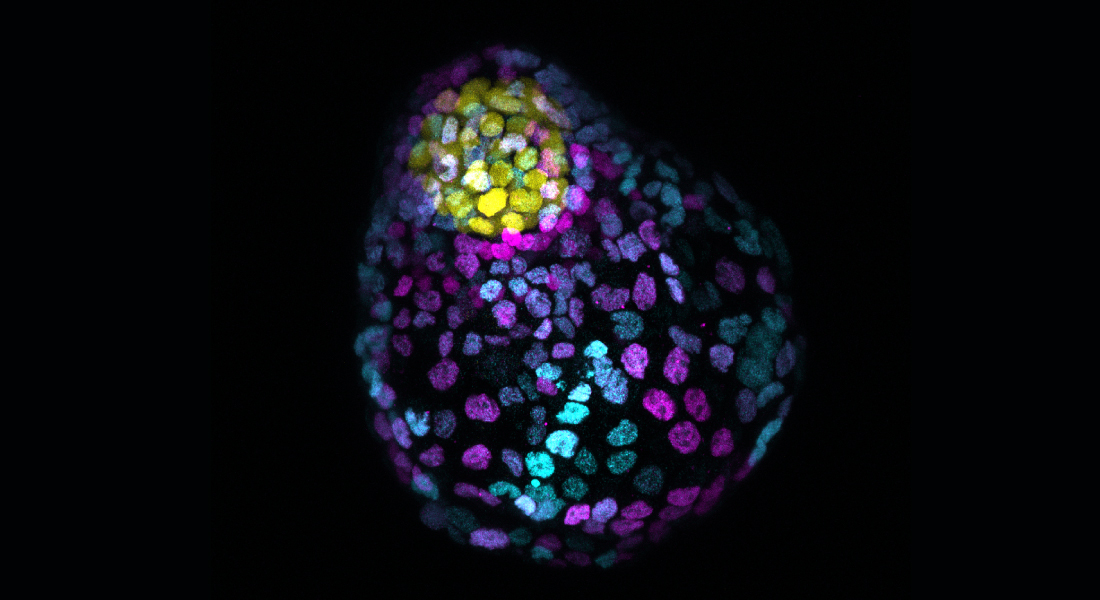
These cells, and the in vitro-cultured stem cells derived from them, remember how to make a complete embryo and have the capacity to recapitulate embryonic development in the absence of other cell types.
The Brickman group at reNEW, Copenhagen node, led by Professor Joshua Brickman, are interested in understanding the origins of biological patterns, particularly at genome level. The genome relates to the complete set of genes or genetic material present in a cell or organism and the group is researching how cells access the information in our genomes.
Leading up to the publication of this paper, PhD student Madeleine Linneberg-Agerholm, and colleagues, found that a cell type in the early embryo maintains a window of plasticity during which time it is possible to redirect the fate of the cells and make any other cell type. Plasticity here relates to the ability of cells to change their phenotypes in response to signalling cues and environmental disturbances.
“The preimplantation embryo has three cell types; the epiblast, the trophoblast, and the primitive endoderm. These three cell types are formed as a result of two binary cell fate decisions. It begins with a single cell which divides to produce two identical cells which then divide into four, then eight, etc., before the outer cells become polarised and develop into the trophoblast,” said Professor Brickman.
The trophoblast cells then make the placenta and the inner cells are at that point called the inner cell mass. The inner cell mass will then decide to make either the primitive endoderm, which will later form the yolk sac, or the epiblast, which gives rise to the embryo itself. The primitive endoderm (or hypoblast in humans) is the last of these two cell types to be fully specified and using an in vitro stem cell model for these cells that the Brickman group developed several years ago, in this paper they found that these can develop into a stem cell-based embryo model referred to as a Blastoid.
The Brickman group already knew from a paper they published in Nature in 2019, that transcription factors stay associated with stem cell genes following the onset of differentiation. As a result of these transcription factors being present, cells remember how to access these genes again, recalling their past identity and upon receiving the right signals, return to their stem cell state. This means that even if a cell has been instructed to adopt a new identity or function, it still remembers what came before.
“It is really quite amazing how the primitive endoderm, that otherwise normally provides nutrient support to the developing embryo, maintains such a high degree of plasticity and cellular memory,” said Madeleine Linneberg-Agerholm.
“This could be particularly important for improving current treatments for infertility, as plasticity and robustness may be the secret to enabling embryos to survive the abnormal environmental conditions found in the laboratory. In support of this idea and our findings, is the recent observation that the primitive endoderm is the main signature of high grade clinical human embryos used in IVF,” continued Professor Brickman.
This was a remarkable discovery and, in fact, was based on a classical experiment done in the 1970s, in which the trophoblast was removed from the embryo and shown to grow back again. Now, over 40 years on, Madeleine has demonstrated, for the first time in history, that the source of this regrowth was the primitive endoderm and that a mouse can be made entirely from extra embryonic cells; cells that primarily give rise to structures that support the embryo during its development.
Read the full paper in Cell here.
