Groundbreaking work on further development of a cellular therapy to treat diabetes
Patients with type one diabetes are currently treated with insulin injections, but there are limitations with this type of treatment. Assistant Professor Philip Seymour, former Assistant Professor Nina Funa and PhD student Heidi Mjøseng, with colleagues from the Serup Group at the Novo Nordisk Foundation Center for Stem Cell Medicine, reNEW, University of Copenhagen, have had a paper published in Stem Cell Reports investigating further development of a cellular therapy to replace the lost insulin-producing beta cells in type one diabetics.
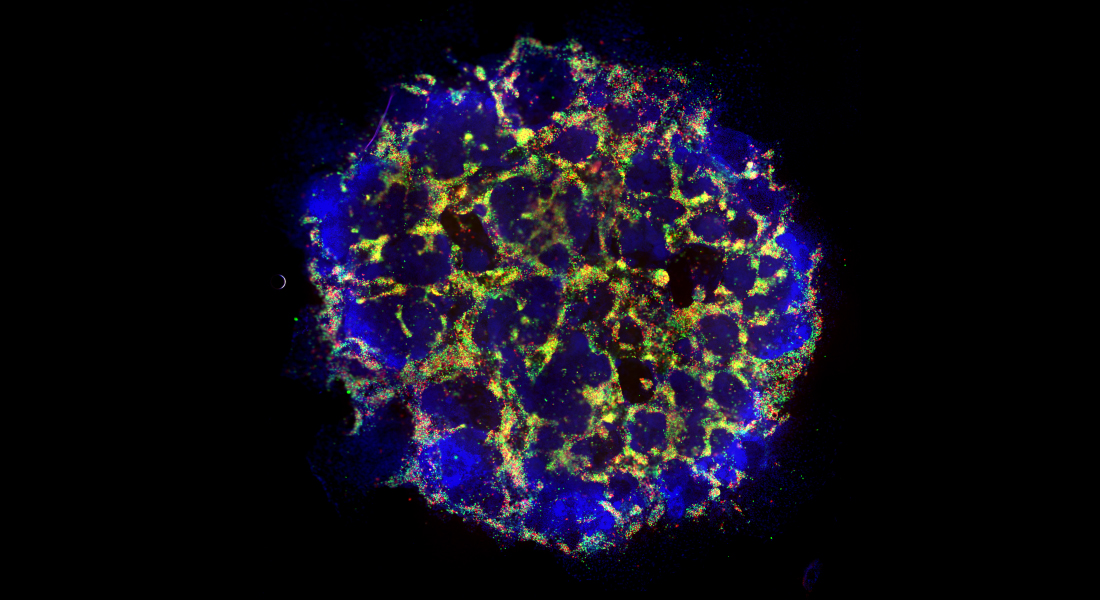
Over 20 years ago, islet transplantation was demonstrated to provide long-term management of symptoms in type one diabetes. Unfortunately, tissue availability remains a major obstacle to this approach: islets - containing insulin-producing beta cells - from two donor pancreases are required for each transplantation. Stem cells, however, hold the promise of being able to generate almost unlimited numbers of beta cells for transplantation.
“The problem is that, although the beta cells that are being produced right now are getting better all the time, they are still not completely matching the function of native beta cells in humans. We also need to find more efficient ways to produce beta cells from stem cells to create the numbers we will need to take this cellular therapy forward,” said Dr Seymour.
If you make stem cells into early gut cells, they can then become either pancreas, liver or intestinal tissue. Former Assistant Professor Nina Funa initially screened for different factors which push stem cells towards becoming pancreas cells instead of liver or intestinal cells. Those “hits” led her to delve into two signaling pathways involved in the pancreas vs. liver “fate” decision of early gut cells; the Wnt and TGF-β pathways. What this paper investigates is how those pathways interact with each other in this process.
“If we inhibit Wnt early on when the cells are becoming pancreas cells, or if we promote TGF-β we can actually make high numbers of insulin cells every time, with every differentiation, which should be really helpful for producing beta cells with possible transplantation purposes in the future,” said Dr Seymour.
A key finding of this paper is that inhibition of Wnt or activation of TGF-β signaling not only makes more pancreas cells, but also drives those pancreas cells into becoming insulin-producing beta cells more efficiently. In addition, the new study shows that intrinsic Wnt signaling activity varies between stem cell “lines”, making some more predisposed to becoming pancreas than others. This is a breakthrough for bringing this type of cellular therapy forward.
“We try to understand the signals, and this has brought forward our understanding of exactly what they need to see to drive home this beta cell fate. The more we can work out precisely which signals are required, the more we can bring down the cost of creating a therapy of this kind,” said co-first-author Heidi Mjøseng.
Responsible for this paper, the Serup Group at Copenhagen University was led by Professor Palle Serup. Professor Serup worked on the pancreas over many decades of his career until passing away after illness in December 2023. The publication of this paper would not have been possible without his generous mentorship, unparalleled knowledge and passionate commitment to his work in this field.
“I think Palle would be very proud that we’ve got this done. This paper forms part of his legacy and it was important for us, from that perspective, to get this work out there for people to see,” said Heidi Mjøseng.
The translational perspectives of this paper, and the findings within, are an exciting development for the field of stem cell research. Not only do they advance a potential cell-based therapy for diabetes, but they also provide key knowledge for other areas of stem cell and developmental biology research.
Read the full paper in Stem Cell Reports here.
