What mechanisms control conversion between fetal and adult intestinal stem cells
It is fascinating to consider how our organs form during development and how they are subsequently replenished during adult life. Just like how we grow from a baby into a child and then into adulthood, organs initially form in an immature state that will go on to mature into fully functional organs. But how is this controlled at the cellular and molecular level? This is one of the questions researchers in the team of Professor Kim Bak Jensen from the Novo Nordisk Foundation of Stem Cell Medicine, reNEW, are addressing in two stories published back-to-back in the journal Science Advances.
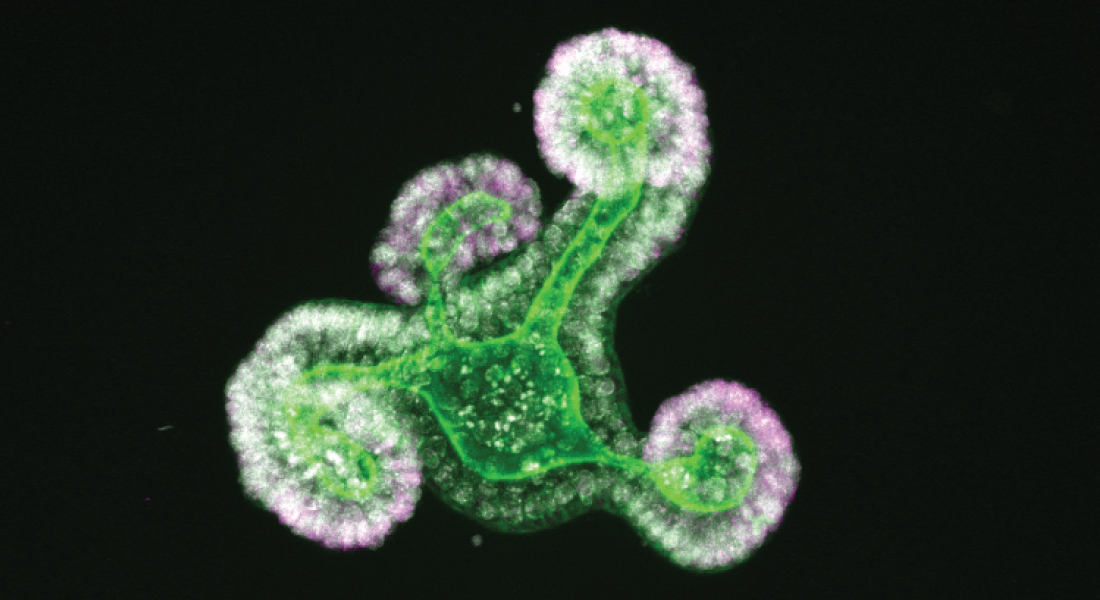
Where it started
The fetal form of intestinal stem cells was initially reported in a seminal paper from the group of Professor Kim Bak Jensen in 2013. Over the last decade, the research group has explored the regenerative potential of these fetal stem cells, their relationship with their adult counterpart, and demonstrated that adult intestinal stem cells following tissue damage revert into a fetal state.
“A greater understanding of the molecular mechanisms that control the transition between fetal and adult stem cells will therefore not only provide knowledge of the process of tissue maturation but also insight into how the process of tissue regeneration is orchestrated,” states Professor Kim Bak Jensen.
The state transition of organs
Organ development and formation are characterized by distinct phases where the organs first go through a state where they grow rapidly by increasing cell numbers and subsequently acquires functionality. In the case of the intestine, this involves acquiring the ability to digest and absorb nutrients. Cell behavior during these distinct phases is controlled at the molecular level by specific transcription factors. These transcription factors are responsible for turning on and off genes, which encode proteins that allow cells to specialize into organ-related stem and differentiated cells. Certain genes, accordingly, play crucial roles in determining how our organs develop and mature.
Professor Kim Bak Jensen and his team investigated specifically how transcriptional factors and related proteins present in the immature cells of the intestine are involved with the transition from an immature to a mature organ. Using two complementary approaches, they identify several genes that control state the transition between a fetal and an adult state in the intestine. They then go on to characterize three of these and show that collectively, the response to biophysical cues maintains cells in a fetal state.
Valuable knowledge for research on other organs
Given that all organs transition between a fetal and adult state, it will be interesting to explore whether the barriers identified in the intestine are shared by other organs. Understanding the state transition from an immature to a mature organ is likely to enable us with insight into how we can coach cells or organ-like structures grown in the lab from immature to mature structures. Such knowledge will ultimately help scientists generate cellular therapies, for instance, via the production of epithelial cells in the lab that can be transplanted into patients with ulcerating conditions or other epithelial defects.
“It is important to keep in mind that, while the potential benefits in this research field are promising and offer exciting possibilities for the future of regenerative medicine, there are still many scientific and technical challenges to overcome,” says Professor Kim Bak Jensen. “Like the two stories we have just published, moving the field forward will require great team efforts, and I am very proud of how my team has pulled together to make these key discoveries.”
The research has been supported by grants from the Novo Nordisk Foundation, the European Research Council, the Lundbeck Foundation, and the Marie Skłodowska–Curie fellowship programme from the European Union.
Read the publications
- Transcriptional and epigenomic profiling identifies YAP signaling as a key regulator of intestinal epithelium maturation
