Stem cell therapy for Parkinson’s Disease granted approval for entering clinical trial in Sweden
Associate Professor and Principal Investigator from reNEW UCPH, Agnete Kirkeby brings hope to people with Parkinson’s Disease by being part of a decade long team effort between universities and hospitals in Sweden and the UK.
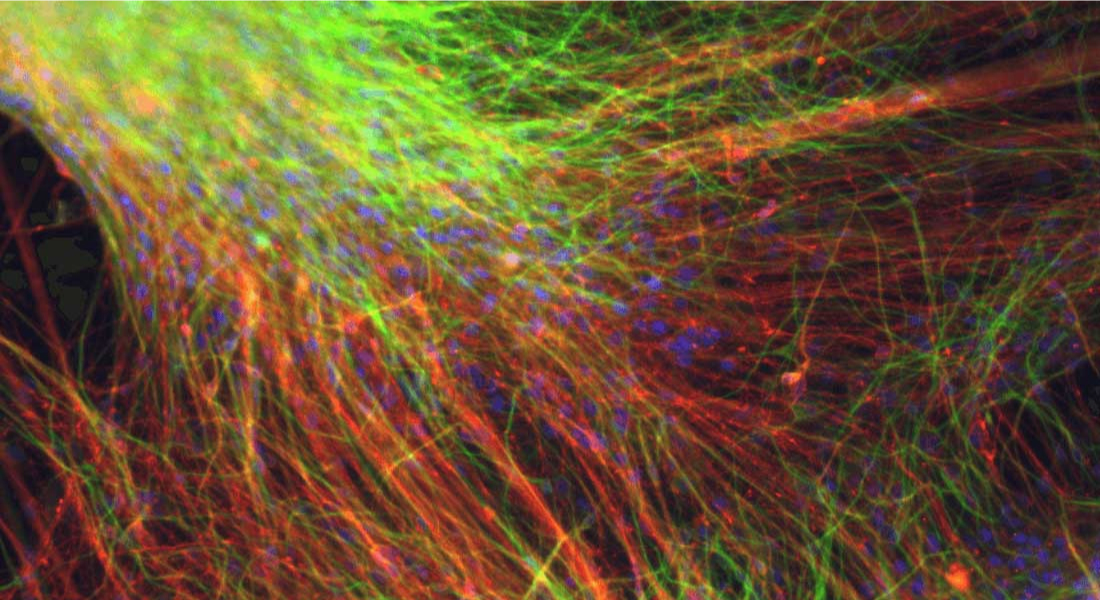
“This achievement is built on work which was started back in 2009 when I started as a postdoc in Malin Parmar’s lab in Lund. After gaining the first positive results from cell transplantations in animal models of Parkinson’s Disease in 2012, we have worked very hard towards making a stem cell therapy to the quality, purity and safety levels suitable for transplantation in humans. Now, we’ve been granted approval from the Swedish authorities to enter clinical trial with this product, which hopefully means that we will soon be transplanting the first patients. It’s very emotional for me to see this project having reached so far”; says Agnete Kirkeby, who is a Principal Investigator at reNEW UCPH and who leads the preclinical development of the stem cell product at Lund University.
The decision from the Swedish Medical Products Agency (MPA) provides regulatory approval for a Phase I/IIa clinical trial for STEM-PD; a human embryonic stem cell based medicinal product for the treatment of Parkinson’s Disease. STEM-PD is a stem cell derived dopamine nerve cell product designed to replace the dopamine cells which are lost in the brains of Parkinson’s disease patients. Ethical approval of the trial has already been obtained from the Swedish Ethics Review Authority, and the STEM-PD team, led by Malin Parmar at Lund University in Sweden, is thereby ready to proceed with the trial.
“Getting stem cell therapies into clinical trial is an enormous task which has been undertaken through an amazing collaboration between our teams in Lund and Cambridge and supported by our close partnership with Novo Nordisk A/S. This is the first pluripotent stem cell therapy for a CNS indication to go into clinical trial in Europe and the first pluripotent-based cell product to be administered to patients in Scandinavia. Our data shows that the STEM-PD product is safe and highly efficacious in reverting motor deficits in preclinical models of Parkinson’s Disease. We hope that cell-based therapies such as STEM-PD can provide patients with novel restorative treatment options to yield better and more long-term symptomatic relief and to minimize their dependency of daily medication”, says Agnete Kirkeby.
National and EU funding agencies has funded the preclinical and clinical studies of STEM-PD. In addition, the STEM-PD team has obtained funding and valuable support for the current study from Novo Nordisk; a collaboration which will continue for future product development.
Read the entire press release from Lund University here.
Please see also:
The German documentary on the possible stem cell treatment for Parkinson’s disease where Agnete Kirkeby has been interviewed: https://gebrueder-beetz.de/en/produktionen/parkinsons-research-brings-hope/
The article 'Forskere vil kurere Parkinsons sygdom ved at sprøjte stamceller ind i hjernen.' published in the newspaper Politiken on November 13, 2022.
